
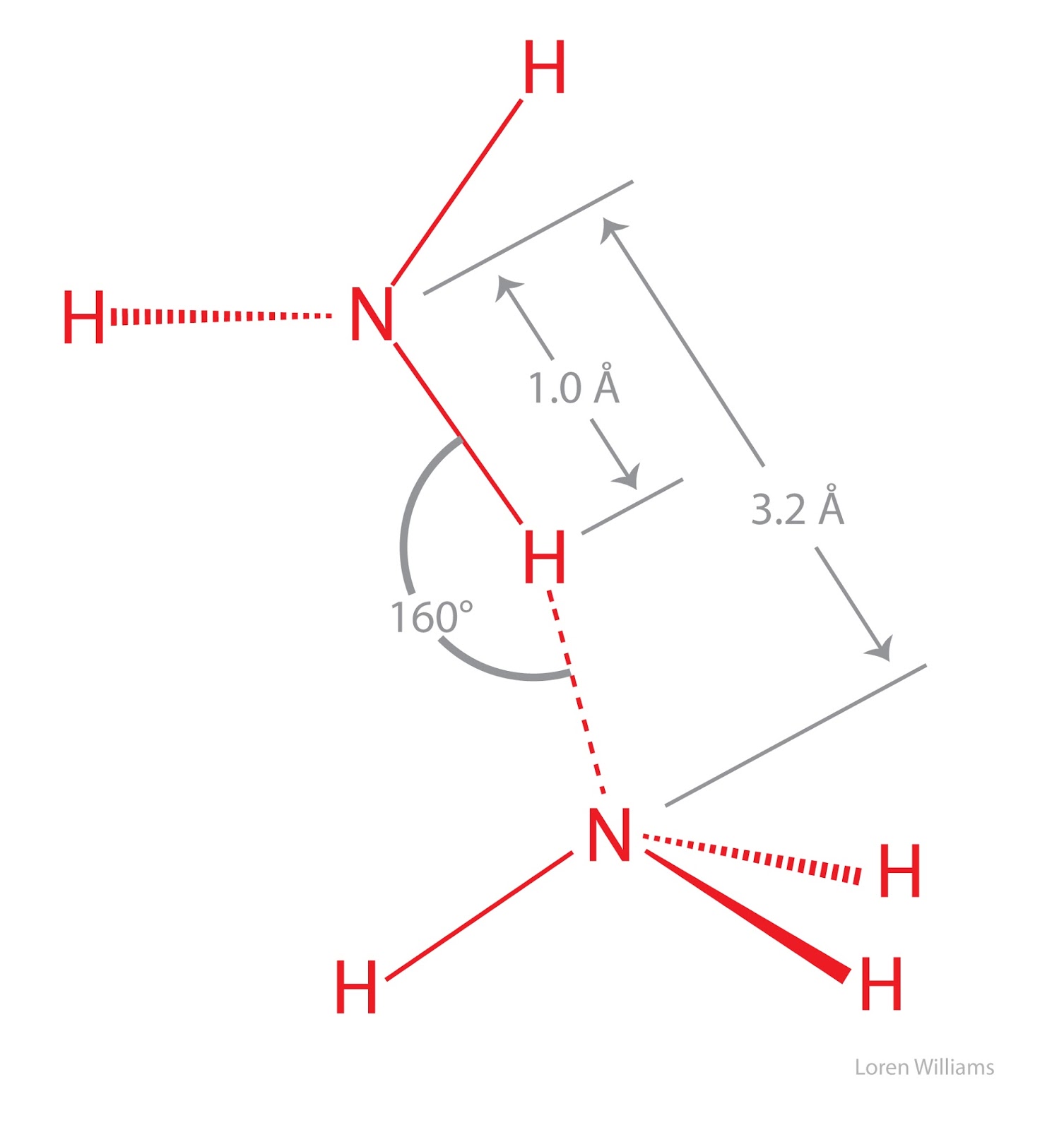
Each substance has its own heat of fusion, and water's heat of fusion is 79.7 cal/g. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors.
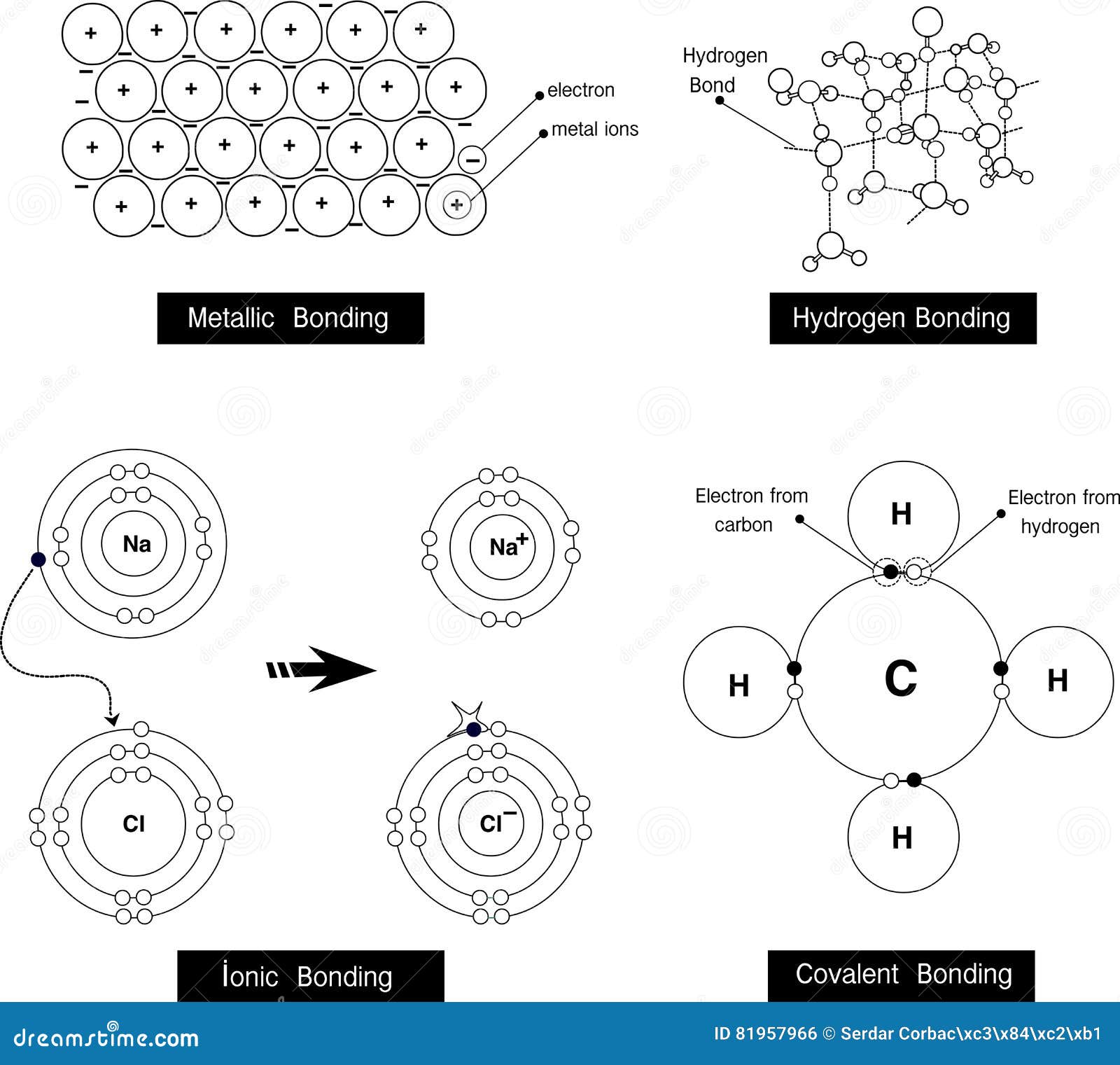
#Hydrogen bonding free#
heat is absorbed when a liquid boils because molecules which are held together by mutual attraction in the liquid are jostled free of each other as the gas is formed. It is found in all three states: vapor, liquid, and solid. The enthalpy of vaporization (symbol Hvap), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. In reverse this gives it a very high heat of vaporisation: a lot of energy is required to turn it into a gas. Latent heat of vaporization is a physical property of a substance.

Why does water have a high latent heat? However, with ice, the surface area doesn't change due to its more rigid structure. Why does water have a higher specific heat than steam? (2) Sweating helps to keep our body cool during hot summer days. Heat of vaporization of water That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. The heat of vaporization is defined as the amount of heat needed to turn 1 g of a liquid into a vapor, without a rise in the temperature of the liquid. Bubbling effect is not visible in evaporation. This property of water helps to cool down the body of living organisms. For a given pressure, different liquids will boil at different temperatures. This amount of heat is calledheat of vaporization. Analytical cookies are used to understand how visitors interact with the website. Their melting points are correspondingly lower at -182C, -78C, and -86C, respectively (Mathews and van Holde 1990). Net evaporation occurs when the rate of evaporation exceeds the rate of condensation. Water may cool air at night and warm during the day. 3 Why is the latent heat of vaporization so much higher than the latent heat of melting? This cookie is set by GDPR Cookie Consent plugin. It has a high latent hit because it take allot of This cookie is set by GDPR Cookie Consent plugin. The amount of heat needed to be added to a substance from the environment. That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. Vaporization is a process in which, the liquid is converted into vapour at its boiling point. Plug these into the equation and solve for q. This cookie is set by GDPR Cookie Consent plugin. Why the latent heat of vaporization of steam is more than that of boiling water? Latent heat, also called heat of transformation, is the heat given up or absorbed by a unit mass of a substance as it changes from a solid to a liquid, from a liquid to a gas, or the reverse of either of these changes. The weaker the bond between atoms, the less energy is needed to break those bonds. water Liquid water has one of the highest specific heat capacities among common substances, about 4184 Jkg 1K 1 at 20 C but that of ice, just below 0 C, is only 2093 Jkg 1K 1. Multiply by 4.0 g on both sides so you can get q by itself. These cookies will be stored in your browser only with your consent.


 0 kommentar(er)
0 kommentar(er)
